About This Project
Yes, mosquitoes are flying syringes! They sample human blood as a normal part of their life cycle. We can take advantage of this nasty habit by using infected mosquitoes as a tool for monitoring the risk to human populations. Viral infection level in mosquitoes reflects the infection level in humans (Kilpatrick, 2013). Mosquitoes can also be used to screen for animal reservoirs of Zika. We’ve used this method to screen insects for parasites and are committed to adapting it to screen for Zika.
Ask the Scientists
Join The DiscussionWhat is the context of this research?
Zika was discovered in Uganda in 1947 during surveillance of monkeys in the jungle. Since then, Zika has spread globally, ultimately spreading to South America. In 2015, Zika emerged strongly in Brazil and other Latin American countries. Responding in February of 2016, the World Health Organization declared Zika virus to be a public health emergency due to the virus’s rapid spread and the suspected effects it has on developing fetuses. Zika is believed to be a zoonotic infection transmitted from wildlife to the human population by a mosquito vector. While mosquitoes transmit many terrible diseases, and most view these creatures as an abominable nuisance, we have developed a method for taking advantage of their blood sucking behavior by screening them for the presence of pathogens.
What is the significance of this project?
This assay could be used to not only screen for Zika, but ultimately many mosquito-borne diseases. With the rapid spread of Zika virus, and the extremely high likelihood of spread of the virus globally, designing a simple, non-invasive, rapid screening method is essential to understanding where the virus is spreading and who is at risk of the disease. The CDC Director has predicted “that Puerto Rico and the U.S. Virgin Islands could have many infections...we will certainly see U.S. travelers returning with Zika infections”. Furthermore, this disease shows the potential to be wide spread ultimately resulting in a global pandemic (see below**). Being able to effectively track this virus as it spreads is essential for preventing massive outbreaks. Listen to Peter Hotez speak about Zika here!
What are the goals of the project?
We aim to develop an assay to quickly and reliably screen for the presence of Zika virus in a certain area. We plan to:
1) identify regions of the Zika virus genome that are highly conserved in Zika, but not in related viruses. We will use these regions for design of a diagnostic assay using quantitative reverse transcriptase PCR.
2) validate this diagnostic on multiple isolates of the Zika virus RNA, and ensuring its specificity by testing on the RNA of other flaviviruses.
3) collect and screen mosquitoes from afflicted areas.
4) make the diagnostic field-friendly and available to those who need it most in endemic areas.
Budget
We need Zika RNA** to test our diagnostic assay. We also need to collect samples from a Zika virus-endemic area in order to validate our assay under field conditions. Additionally we need the reagents to design the quantitative polymerase chain reaction (qPCR) assay. This assay will be capable of detecting even the slightest amount of Zika RNA in a mosquito sample. With no current funding for the development of this assay or the collection of Zika-infected mosquitoes, we will be unable to rapidly design and implement our assay. Traditional sources of funding (grants from the NIH or other agencies) typically take one year from development of a research concept to having the funds available. This is simply too long given the rapid spread of this insidious disease.
**Potential sources of Zika RNA (for controls) are: Medlab Asia Pacific: Vircell Microbiologists (Amplirun® Zika Virus RNA Control) OR Zeptometrix Corporation (Inactivated Zika Virus).
Endorsed by
Meet the Team
Affiliates
Affiliates
Affiliates
Team Bio
Our team is composed of an incredible bioinformatician, three driven molecular parasitologists (human), one veterinary parastiologist, and one extremely dedicated and enthusiastic PI. Our group contains both technicians and graduate students. While we may come from different backgrounds, we represent some of the most senior lab hands in the Williams laboratory, and have come together in the face of this emerging infection. To learn more about our lab please check out our lab website!
Caroline Keroack
I am currently a MSc candidate in the Williams laboratory at Smith College, focusing on surveying the biodiversity of marine mammal parasitic infections and using evolutionary and molecular information to generate important diagnostic tools for these infections. I have a strong passion for infectious disease and have worked on a variety of projects including those focusing on lymphatic filariasis. Although my current focus is mainly on veterinary pathogens (specifically parasites of marine mammals), I am able to bring a unique perspective to many problems in infectious disease. I am very interested in expanding my research to understand zoonotic events.
Weam I. Zaky
Weam is currently a Research Associate in the Williams lab at Smith College. Weam received her Master’s degree at Smith College with Dr. Steven A. Williams. She works on several projects in the lab such as gene expression studies of Wolbachia, RNA-seq projects to study gene expression, and generating and refining molecular diagnostics for neglected tropical diseases. Weam is also our resident entomologist, maintaining different mosquito species in an insectory, identifying and meticulously dissecting mosquitoes, making her our very own mosquito queen! Weam has worked on an extremely wide variety of projects and has an impressive skill set in both the lab and the field. Weam also has experience in the field collecting mosquitoes- an essential part of our project. She is involved in two projects in Haiti screening and collecting data for the prevalence of lymphatic filariasis. Originally from Egypt, Weam brings a unique perspective to the team.
Marina Papaiakovou
Marina is currently a Research Associate in the Williams laboratory at Smith College. After receiving her Bachelor's degree in Greece, Marina moved to the U.S to obtain her Master’s degree at Smith College working on repeat-based diagnostics for soil transmitted helminths (STH). After receiving her Master's, she decided to stay in the lab to continue her work on molecular diagnostics and travel to Argentina (Salta and Orán) conducting field work for the STH diagnostic project. Marina is an avid hiker, a boxer, outdoorsman, beginner climber, and in general is the lab athlete. Originally from Greece (as she says "it's all Greek to her!"), Marina brings creativity, spark and passion to our team.
Jessica Grant
Jessica is currently a Research Associate in the Williams’ lab at Smith College. She received her Master's degree at Smith College with Dr. Laura Katz, with a focus on lateral gene transfer in the parasite Entamoeba histolytica. Jessica was then recruited to the Williams’ lab for her incredible bioinformatic prowess. Jessica is interested in data science and the application of bioinformatics to address various biological questions, including genomics, molecular evolution, and microbiome research. Jessica is also an excellent baker, and makes professional-caliber cakes!
Nils Pilotte
Nils is currently a Research Associate and Ph.D. candidate in the Williams laboratory at Smith College. Nils’ research focus is primarily on the generation of quality molecular diagnostics for neglected tropical diseases. Nils has been involved in many major field studies, supported by the Bill and Melinda Gates Foundation, has received Gates Foundation funding to develop better molecular xenomonitoring tools, and researches infections ranging from the STHs, to lymphatic filariasis, to onchocerciasis, and more. Nils brings great experience and knowledge about the development of diagnostics to the team.
Steven A Williams
I've been a faculty member at both Smith College and the University of Massachusetts for the past 30 years. I'm also an adviser for the Task Force on Global Health and an active participant in the Global Programme to Eliminate Lymphatic Filariasis. My research focus is to elucidate the molecular biology of the parasites that cause elephantiasis, African river blindness and other neglected tropical diseases (NTDs). Most of the research in my laboratory is focused on the goal of eliminating these diseases that afflict an estimated 1.5 billion people worldwide. Specifically, we work on advanced DNA diagnostics and genomics. Recently, a talented group in my lab has expanded our diagnostic efforts to include viruses that infect humans such as dengue, chikungunya and zika. We also have an excellent group in the laboratory that studies the parasites of endangered marine and terrestrial mammals. Our research is funded primarily by the Bill and Melinda Gates Foundation and the National Institutes of Health.
Additional Information
**Potential for Zika spread, NEJM
**Potential spread of Zika, JAMA
**BBC commentary on spread of Zika (reports from World Health Organization)
Recently (3/10/16) the New York Times published the following article which states:
"Since Zika is harmless to most people infected by it, Dr. Kieny [an assistant director general at the World Health Organization] said, “the most pressing need” at this stage of the response is developing diagnostic and preventive tools, in particular tests that could distinguish Zika from related diseases like dengue and chikungunya"
This nicely highlights the need for an assay like ours!
Using similar methods, our lab has already developed successful molecular diagnostic assays for a large number of devastating neglected tropical diseases, including the mosquito-borne parasites that cause lymphatic filariasis and elephantiasis and the soil-transmitted helminth parasites (hookworm, roundworm, whipworm and others). These diseases, together, infect over 1 billion people worldwide and cause unimaginable levels of human misery and economic hardship. More recently, we have begun work towards developing similar DNA assays for seal heartworm (an important infection of harbor seals). We are also currently generating molecular diagnostics for dengue fever and Chikungunya, thus highlighting our expertise in this area. Funding for flavivirus research has traditionally been very limited, and federal grants take time to process, time we do not have given the current rates of Zika transmission. We are reaching out to all of you in the community to help us achieve our goal of producing a gold-standard tool for Zika xenomonitoring (mosquito screening for virus) within 6 months. Our group combines the power of bioinformatics and molecular biology with extensive knowledge of both veterinary and human diseases, allowing us to tap into great creativity, collaboration, and critical thinking to aid in the fight against global problems like the spread of Zika.
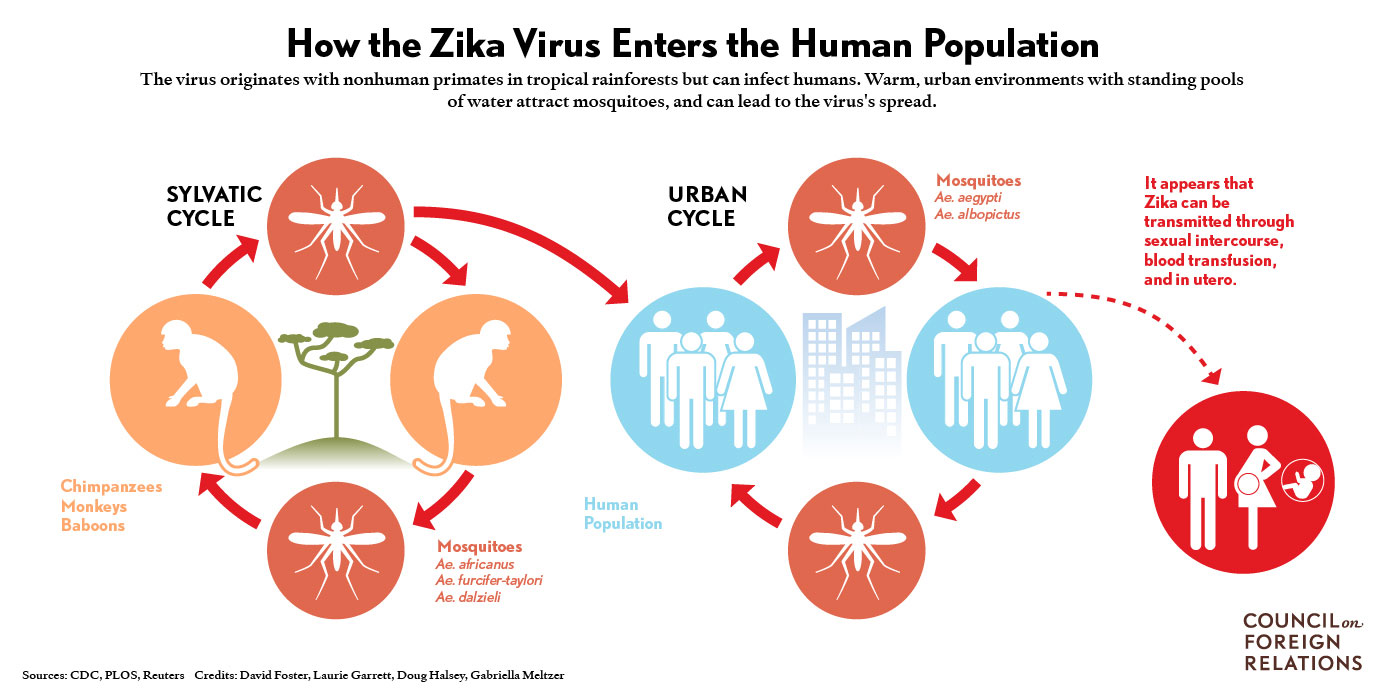 1) Schematic representation taken from the CDC of the perpetuation of Zika virus in humans and animal reservoirs.
1) Schematic representation taken from the CDC of the perpetuation of Zika virus in humans and animal reservoirs.
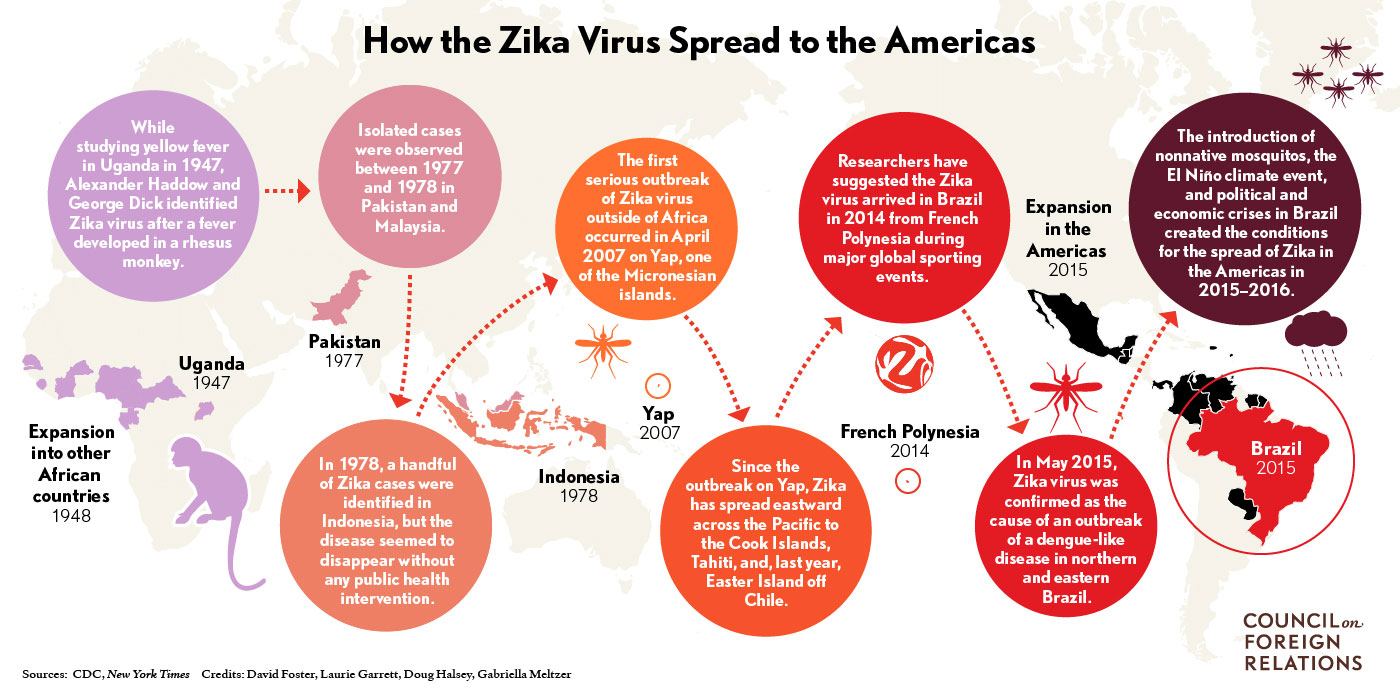 2) Schematic explanation provided by the CDC explaining the global spread of Zika virus.
2) Schematic explanation provided by the CDC explaining the global spread of Zika virus.
Project Backers
- 127Backers
- 144%Funded
- $4,328Total Donations
- $34.08Average Donation











