About This Project
Insulin and exercise improves diabetes, AD and PD. In diabetes, resident immune cells (RICs) secrete inflammatory cytokines upon ingestion of fat deposits, inhibiting Akt signaling. Glial derived neurotrophic factor (GDNF) also activates Akt. Perhaps a similar mechanism occurs in the CNS, involving RIC exposure to alpha-synuclein aggregates. We will attempt to replicate this in vitro and characterize RIC inflammatory status, then expose neuronal lines to RIC and monitor Akt inhibition.
Ask the Scientists
Join The DiscussionWhat is the context of this research?
Studies have suggested that dementia has a possible connection to diabetes. A major component of type 2 diabetes, RICs secrete inflammatory signals that oppose the action of insulin. Due to my experience with peripheral neurodegeneration, I was offered an opportunity to design a project focusing on central neurodegeneration. This proposal was prompted by other studies which have shown that insulin or exercise corrects peripheral diabetes and also a number of neurodegenerative insults in the CNS. Insulin or neurotrophic factors GDNF activate Akt protein. Loss of Akt activation leads to dysfunction and/or cell death peripherally and centrally, thus Akt is an essential signal of robust trophic support and vitality.
What is the significance of this project?
This study may provide evidence that inflammatory signaling can lead to a inhibition of trophic response within the CNS. Improper trophic response impairs cell function and can lead to cell death. Thus, neurotrophic factor resistance may be viewed as a novel mechanism for neurodegeneration. Therapies can be designed which enhance Akt signal or block the inflammatory signals which inhibit Akt signaling in the CNS. For example, administration of intranasal insulin improves symptoms and pathologies of PD, AD and traumatic brain injury. Lastly, RIC response to alpha-synuclein deposits (aSyn), may suggest a unifying theme for diseases of aggregation. There may be other diseases which have interstitial aggregation in common to their pathologies, a few of which are type2 diabetes, PD, and AD.
What are the goals of the project?
Alpha-synuclein (aSyn) synthetic aggregates like the aSyn deposits found in PD (PDf) or control aSyn (CTf) will be applied to a microglial cell line (uG). We expect that PDf treated uG cells to become inflammatory, and that CTf treated cells will change minimally, confirmed via measurement of inflammatory signal. Collected media from the treated uG cells will be applied to a neuronal cell line similar to the neurons which are affected in PD. Trophic signal will be measured by phospho-Akt in neuronal cells basally, or treated with glial derived neurotrophic factor (GDNF). We predict that in GDNF stimulated PDf vs CTf treated neuronal cells, the Akt response will be blunted in PDf compared to CTf uG treated neuronal cells , indicating resistance to trophic stimulus.
Budget
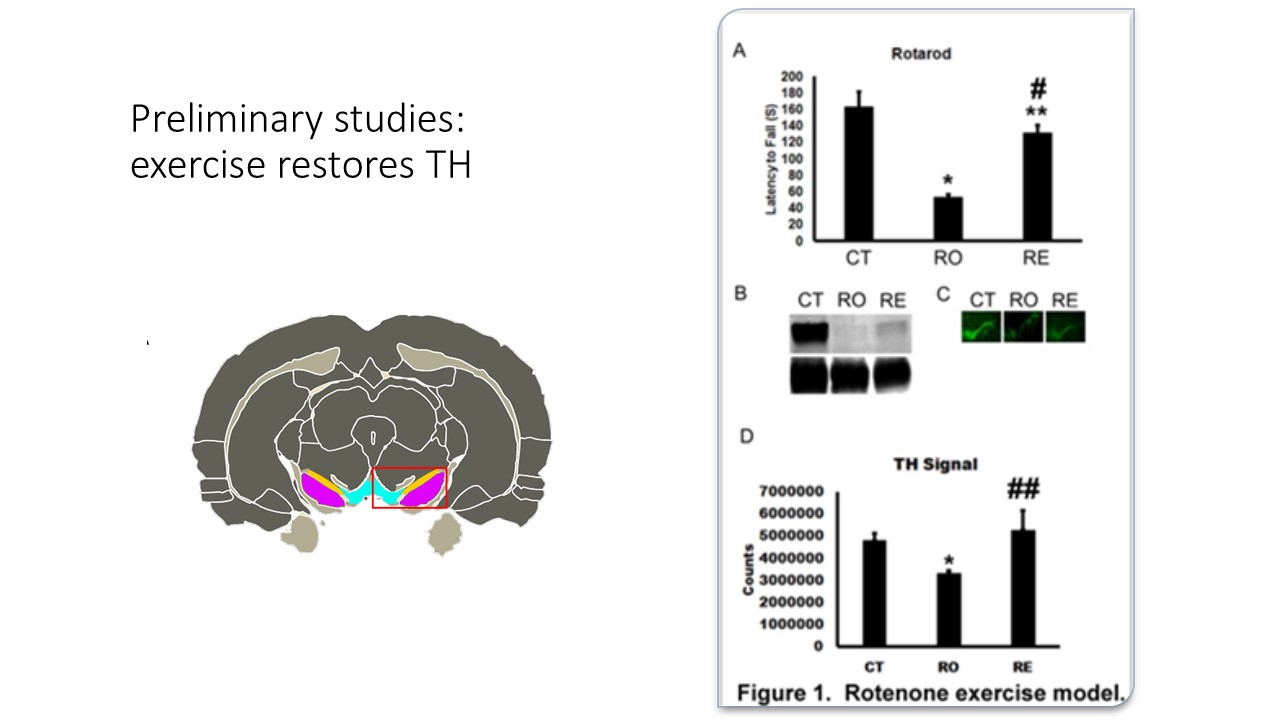
This study is preliminary, this data will be used for a larger in vivo proposal, addressing our hypothesis in greater detail and rigor. We have already observed in vivo the loss of motor coordination and restoration with exercise in a rat model of PD (see additional information). We have evidence which suggests that RICs may be polarized to inflammatory phenotypes in a Parkinson's disease rat model and switched to a restorative phenotype with exercise, a process which is strikingly similar to exercise therapy in diabetics. These funds will be used to show proof of principle in vitro.
Endorsed by
 Project Timeline
Project Timeline
This is roughly a six month timeline to gather the necessary data to show proof of principle using our proposed model. By taking a stepwise, focused approach we expect to be able to find the weaknesses in our methodology and correct them before we move forward in complexity. These time points highlight sharable milestones with our backers and provide transparent evidence of progress.
Dec 13, 2021
Project Launched
Dec 31, 2021
Succesful funding campaign-acquire raw materials
Jan 31, 2022
Establish cell lines and culture system
Feb 28, 2022
Optimize fibril treatments
Mar 31, 2022
Optimize measurement of inflammatory status
Meet the Team
Team Bio
Jason seeks to mentor a sprouting new scientist with these funds. Thus, an undergraduate researcher will be working closely with me, receiving an authentic research experience. With the data gathered, we will apply for an NIH R15 grant which is uniquely suited to strengthen the legacy of undergraduate research at the University of St. Thomas in St. Paul, Minnesota.
Jason S Groshong
Jason studies mechanism of disease, with topics ranging from peripheral and central neurodegeneration, muscle biology, cancer biology, diabetes and obesity. He currently focuses on the parallels between Diabetes, Parkinson's, and Alzheimer's disease, something that has fascinated him for about 20 years now.
Additional Information
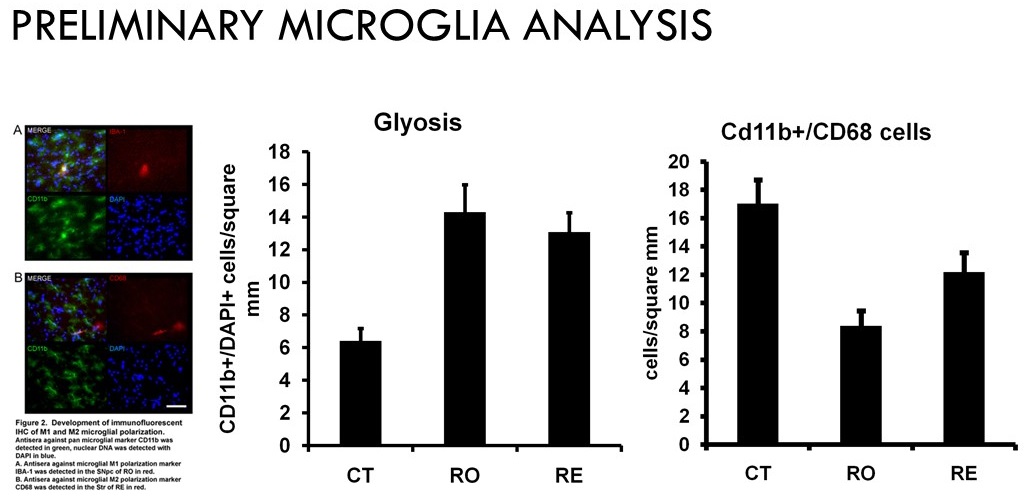
Taken together these results suggest the microglial phenotype changes with rotenone treatment to an inflammatory mode of action (M1), and that exercise improves motor coordination and neuronal function associated with a sparing of a restorative microglial phenotype (M2).
Project Backers
- 16Backers
- 25%Funded
- $2,925Total Donations
- $182.81Average Donation