Methods
Summary
In order to study the anti-tumor effects of our experimental drugs targeting myeloma tumor cells in the bone marrow, we are using a special mouse model. In this model, we have genetically modified human myeloma cell lines
to express the luciferase gene (the gene that is responsible for making
fire-flies glow), and then we inject the cells into specially derived immune-deficient mice. The cancer cells will then migrate and engraft in the bone marrow and form tumors. We can "visualize" the tumors in the bone using special imaging and X-ray technology that measures the light produced by the luciferase gene produced by the cancer cells in the bone marrow -- In the composite picture below, human
luciferase expressing myeloma tumors (the colored spots) are shown to
have engrafted in various parts of the skeleton of 3 different mice. Using this model, we can then study very specific biochemical, molecular, and disease pathways in the tumors and ask how they respond to different chemotherapeutic drugs.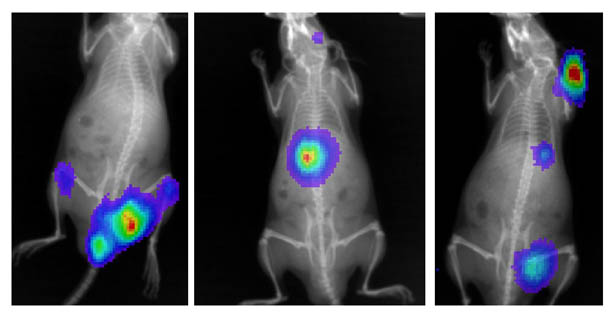
Next, we will use a synthetic DNA binding drug that is designed
to inhibit the ability of an important gene transcription factor called hypoxia-inducible transcription
factor (HIF) from upregulating the expression of certain genes in low oxygen environments, like which exist in the bone morrow. HIF is
actually made up of two subunits, an alpha unit and a beta unit. The
alpha unit is very sensitive to oxygen levels, and under conditions
where oxygen levels are "normal" in the cell, a complex series of
events target and degrade this protein so that it cannot bind to the
beta subunit (see part A in figure below). However, when oxygen levels
are "low", the regulation of the alpha subunit is inhibited, and the two
(alpha and beta) subunits can bind together and activate gene
expression by forming a complex with specific DNA sequences. Our
experimental molecule (called HIF-PA) can bind to the DNA sequences
recognized by HIF and potentially inhibit HIF/DNA binding which will
lead to blocking the expression of the HIF-responsive genes (see part B
in figure below).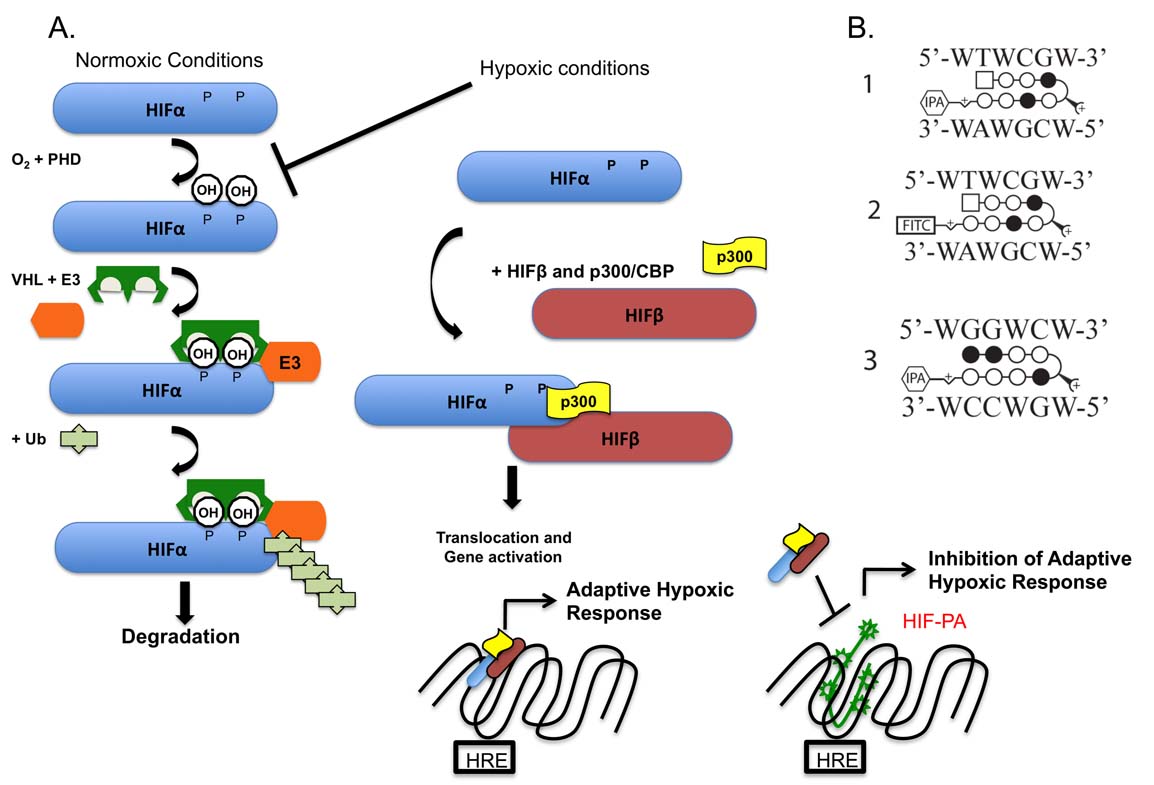
We have recently published our initial results showing that our chemotherapeutic drug is effective at inhibiting the ability of MM cells to live in a hypoxic environment (https://www.ncbi.nlm.nih.gov/p...).
Now we are using this tumor model to study specific aspects of tumor biology, such as how our drugs may effect the metabolism of tumor cells. This is done using PET/CT technology which allows us to use radioactive probes and tracers designed to study specific biological processes, such as the uptake of glucose (the main source of cell energy). In the figure below, the uptake of a radioactive glucose probe (called 18-F-FDG) is shown in mice with two myeloma tumors (T1 and T2). The photo on the right shows the location of the tumors (luciferase positive areas) and the picture on the left shows the same mouse labeled with 18F-FDG (the yellow/orange areas). Normally, the heart (H), brain, kidneys (K) and bladder (B) also uptake the probes, but you can clearly see the probe in T1 and T2. Using this model, we believe we can study the ability of our chemotherapeutic drug to inhibit tumor metabolism.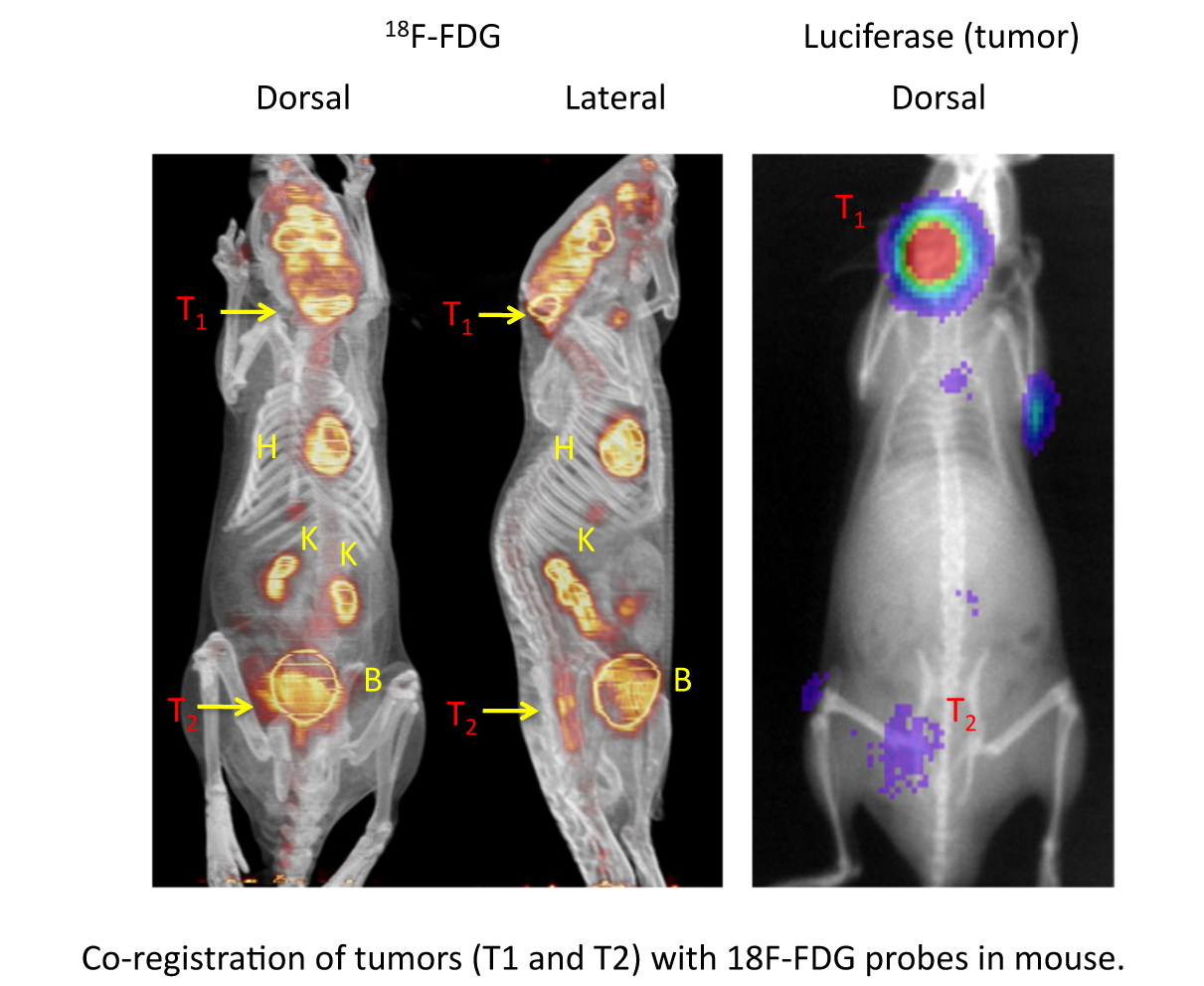
Finally, we are also studying how our drugs may block a painful and deadly effect of tumors in the marrow -- specifically the formation of bone lesions. These painful lesions are likely caused when tumor cells disrupt the normal function of bone cells, resulting in weakening of the bone morphology. We can use PET/CT and a specific probe (18F-NaF) for labeling bone matrix to measure and observe these lesions in the skeleton of mice. We look for areas where the tumors inhibit the uptake of NaF, leaving a void (see arrow) in the bone. In future experiments, we will measure how our drug can inhibit the formation of these lesions over time and study the mechanisms of how this occurs.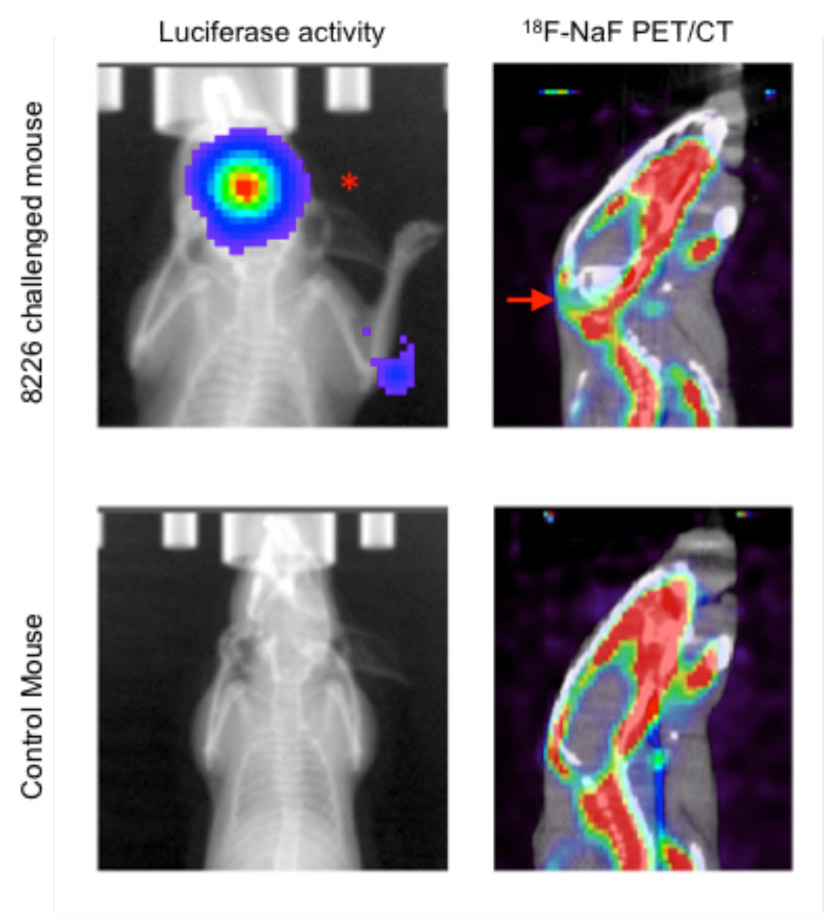
Challenges
As with any new technology, there are many factors that need to be addressed. This includes the best way to treat the mice, the frequency of measurements, and anticipation of unexpected outcomes and results. However, we believe we have a well developed model and a strong scientifically relevant strategy to study the anti-tumor effects of our study drug against myeloma cells engrafted in the bone marrow. One of the strengths of this strategy is that our imaging technology allows us to study the biology of myeloma using real time and non-invasive techniques that are already being used in the clinical setting for treating patients with this disease. Thus, we are well positioned to translate our experiments from the "bench" to the "bedside".
Pre Analysis Plan
Our experimental plan is very simple. We will challenge our mice with tumor cells and once they have formed tumors in the bone marrow, the mice will be treated with our experimental drug or control compounds. Next, we will monitor two aspects of tumor biology using the PET/CT -- changes in metabolism (using 18F-FDG) and changes in lesion formation (using 18F-NaF).
We will test the hypothesis that our experimental drug treatment of MM tumors inhibits FDG uptake and increases NaF incorporation.
Finally, we will use a microCT to study harvested bone for changes in bone lesions. As seen in the picture below, we measured the changes in bone lesions (see red circles) in mice treated with another anti-MM drug.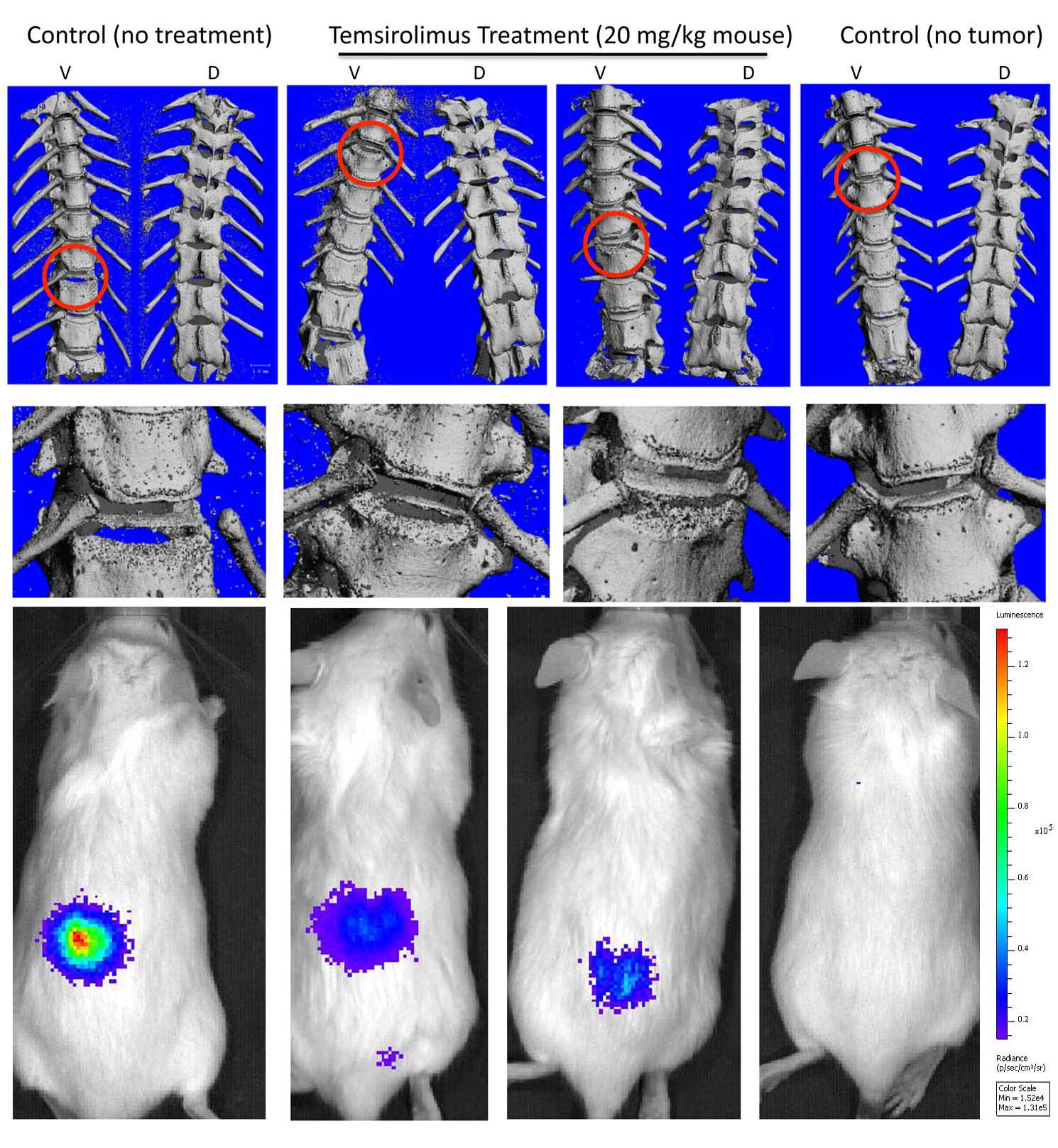
Protocols
This project has not yet shared any protocols.